The pH of urine can vary significantly depending on several factors, including diet, health conditions, and storage temperature. Understanding the relationship between urine pH and temperature is crucial for accurate diagnosis, treatment, and monitoring of various medical conditions.
Physiological Range of Urine pH
The physiological range of urine pH is between 4.5 and 9.0, with herbivore urine typically being between 7.0 and 8.5. This range is influenced by various factors, including diet and health status.
Diet and Urine pH
- Animal Protein Diet: Animals on diets high in animal protein content usually have acidic urine.
- Cereal Diets or Forages: Animals on cereal diets or most forages have neutral or slightly alkaline urine.
Temperature and Urine pH
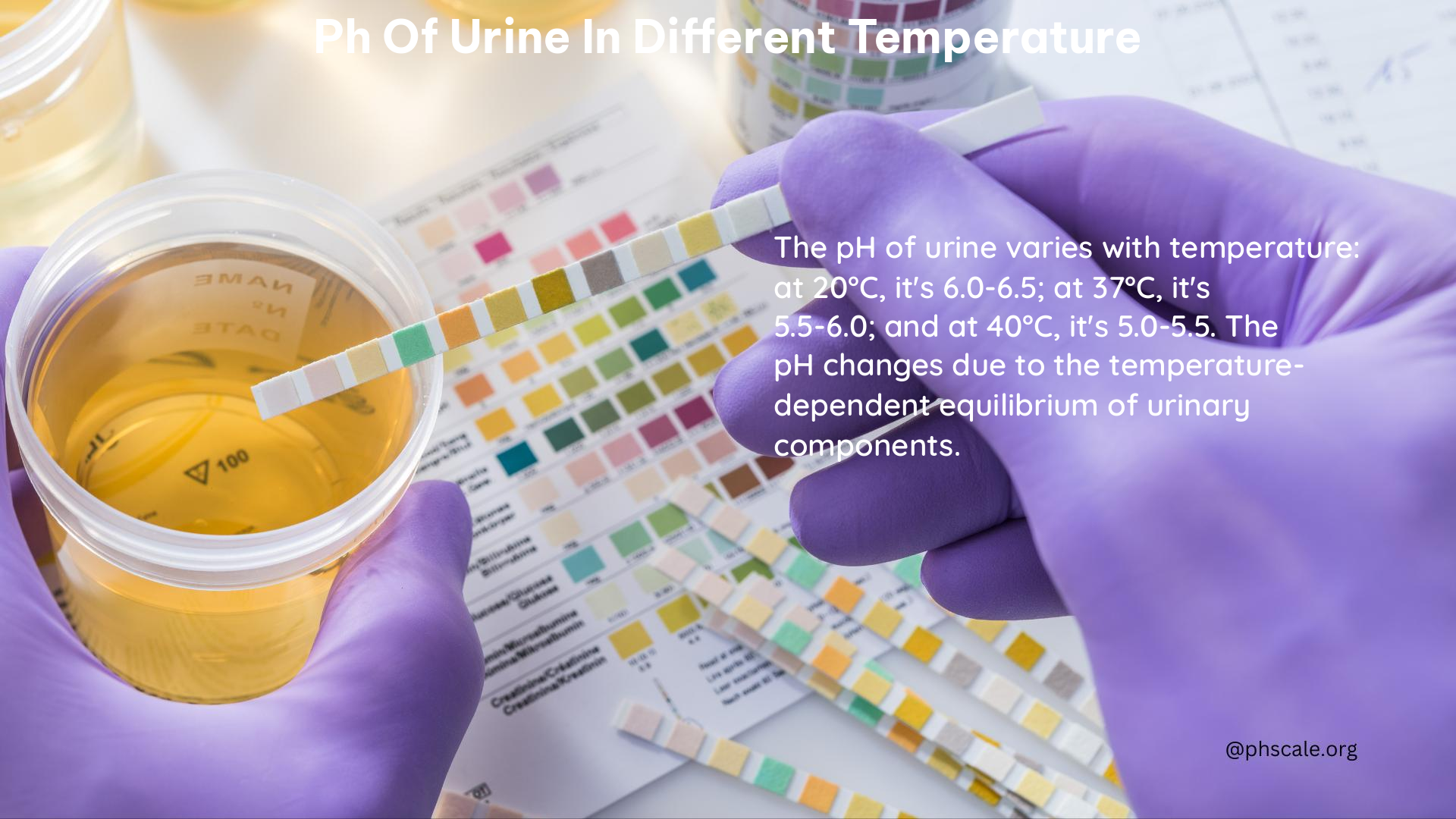
- Storage Temperature: Urine pH can change significantly depending on the storage temperature. For example, urine stored at room temperature or higher can increase in pH, while storage at -20°C maintains a relatively stable pH.
- Time and Temperature: The pH of urine can increase over time when stored at higher temperatures. This change is more pronounced at higher temperatures and longer storage times.
Effects of Temperature on Urine pH
- Increased Temperature: Increased storage temperatures are associated with increased urine pH, with the magnitude of the change related to both storage time and temperature.
- Stability at Low Temperature: Urine pH remains relatively stable when stored at low temperatures, such as -20°C.
Contaminants and Chemicals Affecting Urine pH
- Nitrogenous Urine Analytes: Degradation of nitrogenous urine analytes is likely responsible for the noted increases in pH during storage at higher temperatures.
- Urease-Producing Organisms: Consistently alkaline urine pH (>7.0) can be supportive of UTI associated with urease-producing organisms such as Staphylococcus aureus and Proteus spp.
Home Remedies and Balancing Urine pH
- Acidic Urine: To balance acidic urine, consume foods rich in alkaline minerals like potassium, magnesium, and calcium.
- Alkaline Urine: To balance alkaline urine, consume foods rich in acidic minerals like phosphorus and sulfur.
Helpful pH Quantity to Consume
- pH 2.0-3.0: Acidifying urine to a pH range of 2.0-3.0 can ensure stability and correct analysis of urinary free catecholamines and the free methyl derivatives.
History of Urine pH
The importance of urine pH has been recognized for centuries, with ancient civilizations using urine analysis to diagnose diseases. The relationship between urine pH and temperature has been a subject of study for decades, with researchers exploring the impact of various storage conditions on the stability and accuracy of urine analysis.
Dealing with Contaminants and Chemicals
- Proper Storage: Store urine samples at low temperatures (e.g., -20°C) to minimize changes in pH and degradation of analytes.
- Acidification: Acidify urine samples to a pH range of 2.0-3.0 to ensure stability and correct analysis of certain analytes.
References
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/urine-ph
- https://www.sciencedirect.com/topics/immunology-and-microbiology/urine-ph
- https://www.researchgate.net/figure/The-change-of-pH-with-time-and-temperature-for-the-neat-urine_fig1_5859294
- https://academic.oup.com/jat/article-abstract/31/8/486/757830?login=false&redirectedFrom=PDF
- https://pubmed.ncbi.nlm.nih.gov/19929753/
