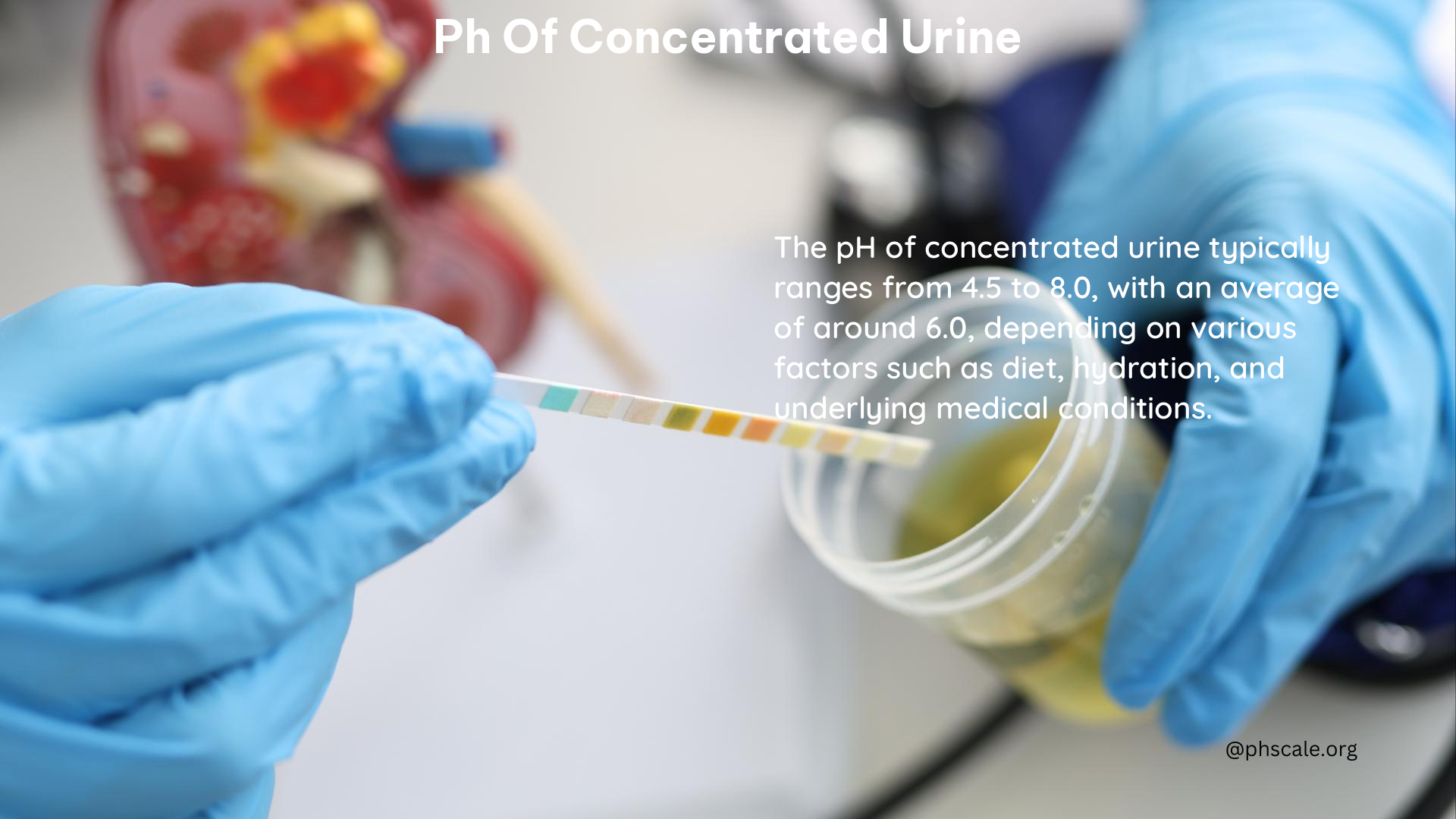
The pH of concentrated urine is typically more acidic than regular urine, with a range typically between 4.5 and 8.0. This acidity is influenced by various factors, including diet, medical conditions, and medications. Understanding the pH of concentrated urine is crucial for maintaining overall urinary health and managing certain medical conditions.
Normal pH Range for Concentrated Urine
The normal pH range for concentrated urine is generally between 4.5 and 8.0, similar to regular urine. However, concentrated urine tends to be more acidic, with a pH closer to 4.5.
Factors Affecting pH of Concentrated Urine
Several factors can influence the pH of concentrated urine:
Diet
A diet high in protein, fish, meat products, and cheese can decrease the pH of concentrated urine, making it more acidic. Conversely, a diet rich in fruits, vegetables, and non-cheese dairy products can increase the pH, making it more alkaline.
Medical Conditions
Certain medical conditions, such as kidney stones, diabetic ketoacidosis, and acidosis, can affect the pH of concentrated urine. For example, kidney stones tend to form in highly acidic or basic environments.
Medications
Some medications, like acetazolamide, ammonium chloride, and thiazide diuretics, can alter the pH of concentrated urine.
Contaminants and Chemicals in Concentrated Urine
Concentrated urine can contain various contaminants and chemicals, including:
- Urea: A waste product that can contribute to the acidity of concentrated urine.
- Ammonium: Produced by bacteria in the urinary tract, ammonium can increase the pH of concentrated urine, making it more alkaline.
- Electrolytes: Concentrated urine contains higher levels of electrolytes like sodium, potassium, and chloride, which can affect its pH.
- Waste Products: Concentrated urine contains higher concentrations of waste products like creatinine, uric acid, and other metabolic byproducts, which can influence its pH.
Balancing pH of Concentrated Urine
To balance the pH of concentrated urine, consider the following:
Dietary Changes
Adjust your diet to include more alkaline-forming foods like fruits, vegetables, and non-cheese dairy products if your concentrated urine is too acidic. Conversely, consume more acidic-forming foods like meat, fish, and cheese if your concentrated urine is too alkaline.
Hydration
Drink plenty of water to dilute the concentration of waste products and electrolytes in your urine, which can help maintain a healthy pH balance.
Medication Management
Consult your doctor about adjusting medications that may be affecting the pH of your concentrated urine.
Helpful pH Quantities to Consume
To maintain a healthy pH balance in concentrated urine, consider consuming the following:
| Food Type | Effect on pH |
|---|---|
| Alkaline-Forming Foods (e.g., citrus fruits, legumes, vegetables) | Helps raise the pH of concentrated urine |
| Acidic-Forming Foods (e.g., meat, fish, cheese) | Helps lower the pH of concentrated urine if it is too alkaline |
History of pH Measurement in Urine
The measurement of pH in urine dates back to the early 20th century. The development of pH-sensitive dyes and litmus paper enabled the creation of dipsticks that could quickly and accurately measure the pH of urine samples. Today, pH measurement is a standard component of urinalysis, helping doctors diagnose and manage various medical conditions.
References
- Mount Sinai. (2023). Urine pH Test. Retrieved from https://www.mountsinai.org/health-library/tests/urine-ph-test
- WebMD. (2023). What to Know About a Urine pH Test. Retrieved from https://www.webmd.com/a-to-z-guides/what-to-know-about-a-urine-ph-test
- ScienceDirect. (2024). Urine pH – an overview. Retrieved from https://www.sciencedirect.com/topics/immunology-and-microbiology/urine-ph
- Healthline. (2021). Urine pH Level Test: Purpose, Procedure, Results & More. Retrieved from https://www.healthline.com/health/urine-ph
