The pH of urine in diabetic ketoacidosis (DKA) is a crucial indicator of the severity of the condition. In DKA, the body breaks down fat for energy instead of glucose, leading to the production of acidic ketone bodies that are excreted in the urine, causing the pH to drop. Understanding the significance, measurement, and role of urine pH in monitoring DKA is essential for effective management of this life-threatening complication of diabetes.
Significance of Urine pH in DKA Diagnosis
Urine pH Range
In DKA, the urine pH is typically acidic, ranging from 5.0 to 6.5. This acidity is due to the presence of ketones, which are produced when the body breaks down fat for energy instead of glucose.
Ketone Bodies
The three main ketone bodies are acetone, beta-hydroxybutyric acid (BHB), and acetoacetic acid. These ketones are produced by the liver and excreted in the urine, contributing to the acidic pH.
Urine Dipstick Testing
A urine dipstick test can detect ketones in the urine, which is a key indicator of DKA. The test is highly positive in patients with DKA.
Measuring Urine pH
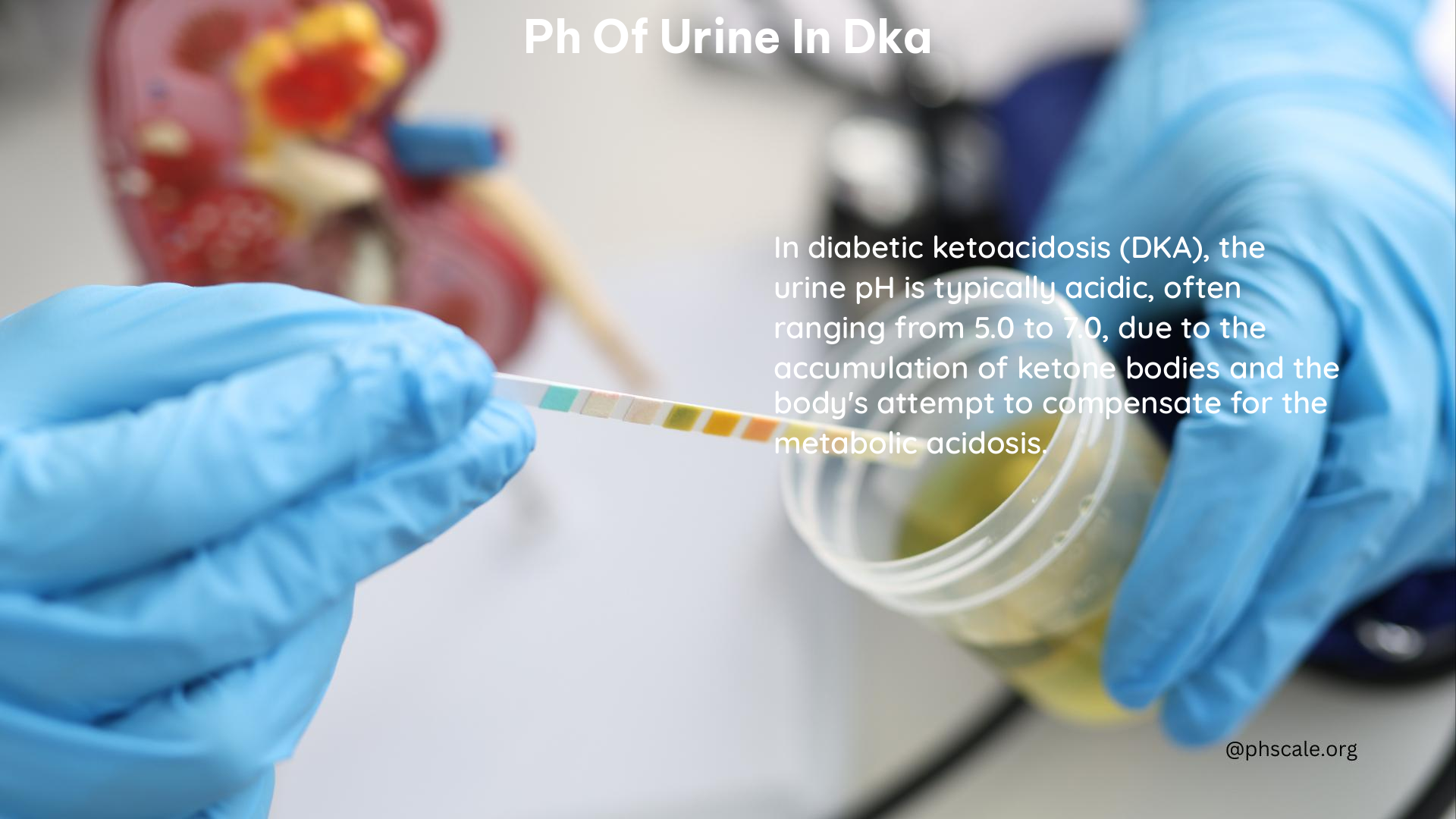
Urine Dipstick
A urine dipstick test can provide a quick and approximate measurement of urine pH. However, it may not be as accurate as other methods.
Laboratory Analysis
Urine pH can be measured more accurately in a laboratory setting using techniques such as titratable acidity (TA) or by measuring the concentration of specific ions like ammonium (NH4+).
Role of Urine pH in Monitoring DKA Treatment
Monitoring Progress
Urine pH can be used to monitor the response to treatment in patients with DKA. As treatment progresses, the urine pH should increase, indicating a decrease in ketone production and an improvement in acid-base balance.
Adjusting Treatment
If the urine pH remains low, it may indicate that the treatment needs to be adjusted, such as increasing insulin doses or providing additional fluids and electrolytes.
Contaminants and Chemicals in Urine pH of DKA
Ketone Bodies
Acetone, BHB, and acetoacetic acid are the primary ketone bodies present in the urine of patients with DKA, contributing to the acidic pH.
Other Electrolytes
Urine pH can be influenced by the presence of other electrolytes like sodium, potassium, calcium, magnesium, and chloride, which can affect the overall acid-base balance.
Home Remedies and Balancing pH
Hydration
Drinking plenty of water can help flush out ketones and other acidic compounds from the body, which can help balance the urine pH.
Electrolyte Balance
Consuming electrolyte-rich foods or supplements, such as potassium, sodium, and magnesium, can help maintain a healthy acid-base balance.
Insulin Management
For patients with diabetes, managing insulin levels and maintaining good glycemic control can help prevent DKA and maintain a healthy urine pH.
History and Prevalence
DKA Prevalence
DKA is a common complication of diabetes, affecting approximately 4-6% of people with diabetes annually.
Historical Context
DKA was first described in the late 19th century, and since then, there have been significant advances in understanding its pathophysiology and treatment.
References
- Cleveland Clinic. (n.d.). Diabetes-Related Ketoacidosis (DKA): Symptoms & Treatment. Retrieved from https://my.clevelandclinic.org/health/diseases/21945-diabetic-ketoacidosis-dka
- Medscape. (2024). Diabetic Ketoacidosis (DKA) Workup. Retrieved from https://emedicine.medscape.com/article/118361-workup
- NCBI. (2015). Severe Ketoacidosis (pH ≤ 6.9) in Type 2 Diabetes. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4491375/
- LWW. (2010). Metabolic Basis for Low Urine pH in Type 2 Diabetes. Retrieved from https://journals.lww.com/cjasn/fulltext/2010/07000/metabolic_basis_for_low_urine_ph_in_type_2.18.aspx
- NCBI. (2009). Metabolic Basis for Low Urine pH in Type 2 Diabetes – PMC – NCBI. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2893060/.
