Lemon juice is known for its high acidity, with an average pH value ranging from 2.0 to 2.6. This low pH is primarily due to the high concentration of citric acid in lemons, typically 5-8%. The acidity of lemon juice is comparable to vinegar but more acidic than most fruits. Understanding the pH of lemon juice is crucial for various applications in cooking, food preservation, and health.
What is the Exact pH Range of Lemon Juice?
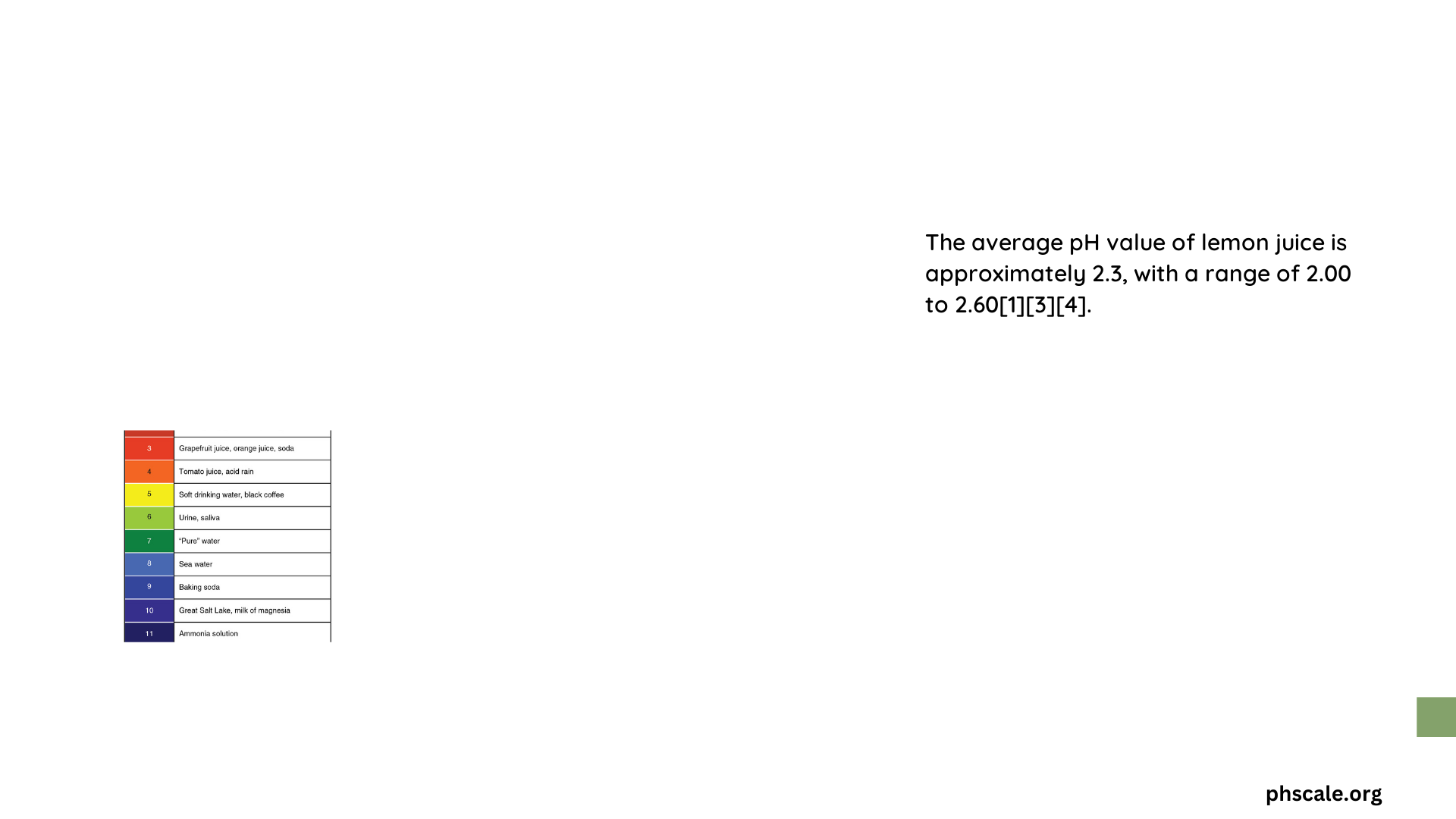
The pH of lemon juice typically falls between 2.0 and 2.6, making it highly acidic. This range can vary slightly depending on factors such as:
- Ripeness of the lemons
- Growing conditions
- Processing methods
- Storage time
To put this in perspective, here’s a comparison of lemon juice pH with other common substances:
| Substance | pH Range |
|---|---|
| Battery Acid | 0 |
| Stomach Acid | 1.5 – 3.5 |
| Lemon Juice | 2.0 – 2.6 |
| Vinegar | 2.4 – 3.4 |
| Orange Juice | 3.3 – 4.2 |
| Coffee | 4.5 – 5.0 |
| Pure Water | 7.0 |
How is the pH of Lemon Juice Measured?
Accurate measurement of lemon juice pH involves several steps and considerations:
-
pH Meters: These electronic devices are the most precise tools for measuring pH. They use a glass electrode to detect hydrogen ion concentration in the solution.
-
Calibration: Before measuring, the pH meter must be calibrated using standard buffer solutions, typically at pH 4, 7, and 10.
-
Sample Preparation: The lemon juice should be freshly squeezed and filtered to remove pulp and seeds.
-
Temperature Control: pH measurements are temperature-dependent, so it’s crucial to maintain a consistent temperature, usually around 25°C (77°F).
-
Multiple Readings: Taking several readings and calculating the average ensures more accurate results.
-
Electrode Care: Proper cleaning and storage of the pH electrode between measurements is essential for maintaining accuracy.
Why is Lemon Juice So Acidic?
The high acidity of lemon juice is primarily due to its citric acid content. Here’s a breakdown of the factors contributing to lemon juice’s acidity:
-
Citric Acid Concentration: Lemons contain 5-8% citric acid by weight, which is significantly higher than most other fruits.
-
Organic Acids: Besides citric acid, lemons also contain small amounts of other organic acids like malic acid and ascorbic acid (Vitamin C).
-
Low Sugar Content: Unlike sweeter fruits, lemons have a low sugar content, which doesn’t offset the acidic taste.
-
Hydrogen Ion Concentration: The high concentration of hydrogen ions (H+) released by these acids results in the low pH value.
What Are the Implications of Lemon Juice’s Low pH?
The low pH of lemon juice has several implications:
- Culinary Uses:
- Acts as a natural preservative
- Prevents browning of cut fruits and vegetables
-
Tenderizes meat by breaking down proteins
-
Health Effects:
- Can erode tooth enamel if consumed frequently
- May exacerbate acid reflux in some individuals
-
Has antibacterial properties due to its acidity
-
Cleaning Applications:
- Effective in removing stains and odors
-
Can be used as a natural disinfectant
-
Chemical Reactions:
- Reacts with baking soda to produce carbon dioxide (useful in baking)
- Can be used to neutralize alkaline substances
How Does Lemon Juice pH Compare to Other Citrus Fruits?
While all citrus fruits are acidic, their pH levels can vary:
| Citrus Fruit | Average pH Range |
|---|---|
| Lemon Juice | 2.0 – 2.6 |
| Lime Juice | 2.0 – 2.8 |
| Grapefruit Juice | 3.0 – 3.5 |
| Orange Juice | 3.3 – 4.2 |
| Tangerine Juice | 3.3 – 4.5 |
Lemon and lime juices are the most acidic among citrus fruits, while oranges and tangerines are less acidic due to their higher sugar content.
Can the pH of Lemon Juice Change Over Time?
Yes, the pH of lemon juice can change over time due to several factors:
-
Oxidation: Exposure to air can lead to oxidation, slightly increasing the pH.
-
Bacterial Growth: If not properly stored, bacteria can grow in the juice, potentially altering its pH.
-
Temperature: Extreme temperatures can affect the chemical composition of the juice, potentially changing its pH.
-
Dilution: Adding water or other substances can raise the pH, making it less acidic.
To maintain the original pH:
- Store lemon juice in an airtight container
- Keep it refrigerated
- Use it within a week of squeezing
What Are the Benefits of Lemon Juice’s Low pH in Food Preservation?
The high acidity of lemon juice makes it an excellent natural preservative:
-
Inhibits Bacterial Growth: Most harmful bacteria cannot survive in highly acidic environments.
-
Prevents Oxidation: The low pH helps prevent the oxidation of other foods, keeping them fresh longer.
-
Enhances Flavor: The acidity can enhance and preserve the flavors of other ingredients.
-
Natural Alternative: It’s a natural preservative option, avoiding artificial additives.
-
Versatility: Can be used in a wide range of foods, from beverages to marinades.
Understanding the average pH value of lemon juice is crucial for its various applications in cooking, food preservation, and even household cleaning. Its high acidity, with a pH ranging from 2.0 to 2.6, makes it one of the most acidic natural substances commonly used in everyday life. This property, while beneficial in many ways, also requires careful consideration in its use and handling.
References:
- MedicineNet: What Is the pH of Lemon Juice, and Is It Considered Acidic or Alkaline?
- Science Notes: What Is the pH of Lemon Juice?
- Cary Institute: Why does a lemon taste tart? Acidity, measured as pH accounts for part of the explanation.
- USDA: pH values of common foods and ingredients
- Journal of Food Science: Citrus Fruit Acidity
